EXPERTISE – A HERITAGE OF EXCELLENCE
OVERVIEW OF COMPLETED PROJECTS
- Clinical operations for more than 450 projects (phase I–IV)
- Medical device investigations (with around 20% of these being first‑in‑human trials)
- More than 30 pediatric studies (including orphan indications)
- Advanced therapy medicinal products (ATMP) trials experience
- Full-service projects
- Auditing services
- Medical writing (Protocols, CSRs, PIL, SmPC, journal papers)
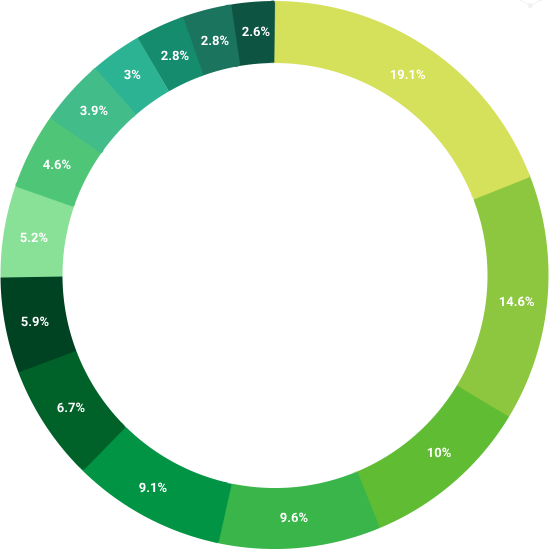
OVER 450 PROJECTS
- 19,1% CNS
- 14.6% Oncology
- 10% Endocrinology
- 9.6% Pneumology & Respiratory
- 9.1% Cardiovascular diseases
- 6.7% Rheumatology
- 5.9% Hepatology
- 5.2% Immunology
- 4.6% Dermatology
- 3.9% Nephrology & Urology
- 3% Gynecology
- 2.8% Ophthalmology
- 2.8% Hematology
- 2.6% Other
| THERAPEUTIC AREA | PHASE I PHASE II |
|---|---|
| Oncology | 9 20 |
| Nephrology / urology / andrology | 4 4 |
| Endocrinology | 2 7 |
| Hepatology / gastroenterology | 2 5 |
| Neurology / pain management | 1 21 |
| Pneumology / respiratory | 1 13 |
| Cardiovascular diseases | 1 10 |
| Dermatology | 12 |
| Other | 5 21 |
| Medical devices – first-in-human | 4 |
EARLY PHASE PROJECTS
Our experienced team comprises highly trained senior clinical research associates and project managers with extensive management experience of early-phase projects across a variety of therapeutic areas.
- Protocol design/writing of first-in-human projects (medical devices as well as medicinal products)
- Experience with healthy volunteers’ projects
- Cooperation with different phase I facilities
You can find more in a short summary of our experience.
PROTOCOL DESIGNS
Our experience includes more than 10 protocol designs (phases I – IV and medical device investigations). Most recently these have included:
- Gastroenterology
- Ophthalmology
- CNS
- Orthopedics
- Dermatology / wound healing
We focus on making our protocols time- and cost-efficient for our clients. Our aim is to provide clear insight into the drug or medical device under study while also employing the minimal patient population through the use of smartly defined endpoints.
Here you will find a short summary of Medical Writing Services and a case study.
- EFFECTIVE PROTOCOL DESIGNS THAT GET APPROVED
- TIME AND COST EFFICIENT
- WORKING TOGETHER TO CREATE MAXIMUM INSIGHT
REGULATORY CONSULTANCIES
Pharmnet protocols have always been well accepted by the regulatory authorities and usually receive only very minor comments.
Pharmnet works to develop 2-3 project approaches which are presented to the client. We will then write the synopsis outline and then develop the full protocol. Should the design not be clear, we will meet with the client to discuss the expected benefit of the product and we’ll explore market expectations. In developing the project, we will seek to mine as much information as possible from the patient data.
MAJOR THERAPEUTIC AREAS
We have rich experience across many therapeutic areas and indications. Details of some of the most frequent are included below.
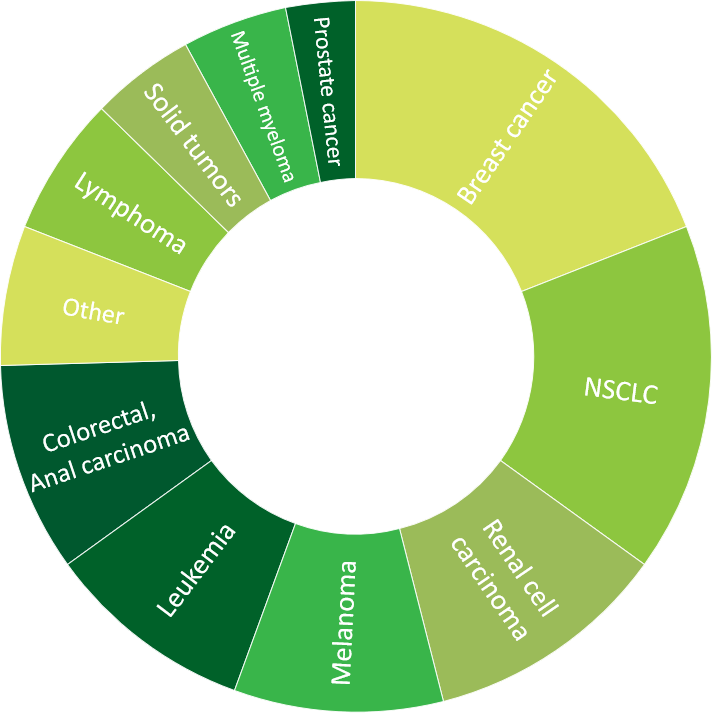
- >60 oncology trials (including 29 phase I/II trials)
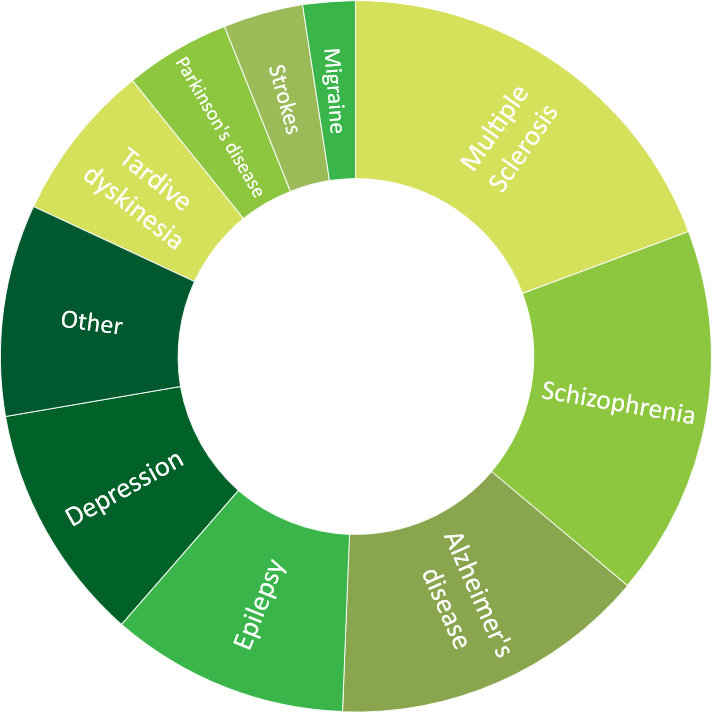
- >80 CNS trials, including phase I trial designs for psychotropic compounds
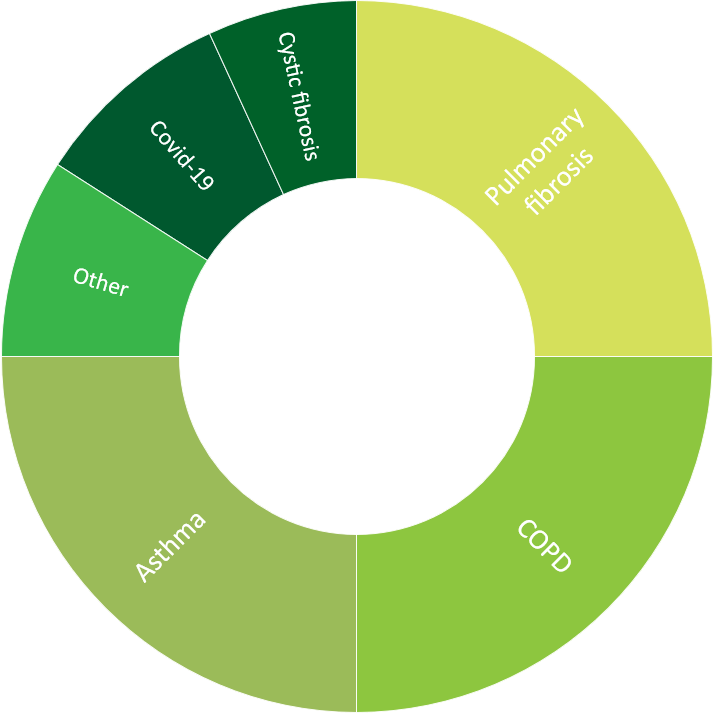
- 44 respiratory projects, including COPD, asthma, pulmonary fibrosis, cystic fibrosis, and systemic sclerosis associated interstitial lung disease, plus additional experience with pulmonary oncology
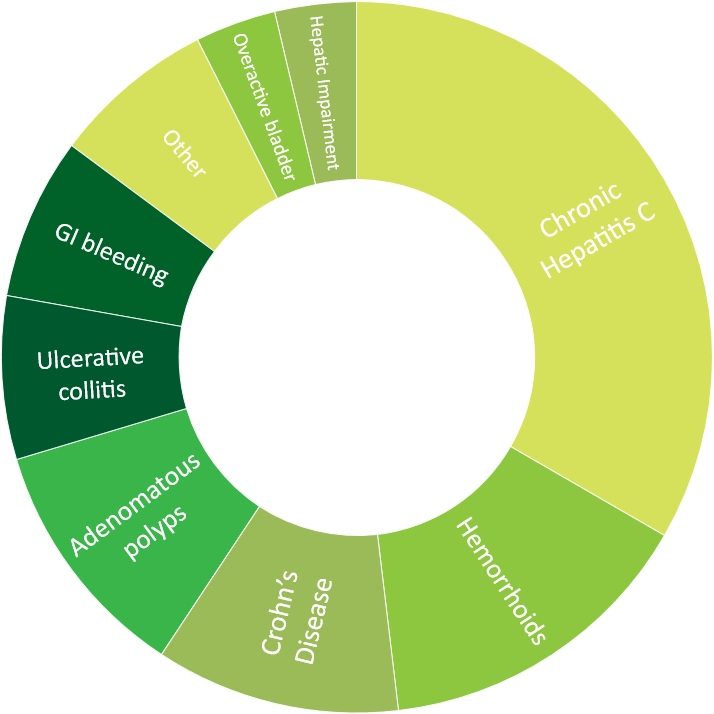
- 27 hepatology and gastroenterology projects, including 2 first-in-human projects
Click to view more detailed information about our experience in oncology, central nervous system, cardiology, ophthalmology, respiratory and metabolic diseases.
If you would like to know more about our experience in your specific area of focus, contact us today.
- RAPID PATIENT RECRUITMENT STRATEGIES
RECRUITMENT RESCUE MISSION
In some projects, we are called on to boost flagging patient recruitment programs. Our approach is to understand as much as we can about the project and then to check which countries and sites will yield the best patient recruitment result. We will also roll out creative ways to keep active sites engaged including building a reference network with other clinicians and organizing local investigator meetings to encourage sites to recruit more patients.
Due to our long-term experience, Pharmnet has a well-established network of sites (more than 450 sites in the CEE region). We can use this to rapidly identify and engage the sites that best suit your therapeutic field of interest. In addition, we have established close relationships with international key opinion leaders, who we consult with for guidance and input.
Click here to read about a case study where we implemented our recruitment rescue strategies.
PEDIATRIC TRIALS
Pediatric clinical trials introduce some unique challenges. However, they are essential for developing age-specific therapies and interventions, and to ensure the best medical treatment can be developed and offered.
- Around 30 pediatric trials completed across various phases (I-IV)
- Various therapeutic areas covered including vaccination, cardiology/cardiovascular, oncology, hematology, respiratory, neurology
Click to view more detailed information about pediatric trials and our experience.
- EXPERIENCED IN THE SPECIAL
REQUIREMENTS OF PEDIATRIC TRIALS